Lithium Fluoride (LiF) is a high-performance optical crystal distinguished by its exceptionally wide transmission range, extending from the deep ultraviolet (VUV/DUV) to the mid-infrared (IR). This rare combination makes LiF an indispensable material for advanced optical systems operating under high radiation, extreme temperatures, or cryogenic conditions.
Compared with other alkali halides, LiF offers lower hygroscopicity, superior UV resistance, and excellent thermal stability, enabling reliable performance in demanding environments.
Key Properties of Lithium Fluoride
- Ultra-Broad Spectral Transmission: High transparency from 105 nm to 6 µm, covering VUV, UV, visible, and IR regions.
- Low Refractive Index: One of the lowest refractive indices among IR materials, minimizing Fresnel losses and optical distortion.
- Excellent UV & Radiation Resistance: Maintains optical performance under intense UV and ionizing radiation exposure.
- Thermal & Cryogenic Stability: Stable optical and mechanical properties at both elevated and extremely low temperatures.
- Low Hygroscopicity: Less moisture-sensitive than most alkali halides, improving durability and handling reliability.
Typical Applications
- UV & VUV Optics: Windows, lenses, and prisms for photolithography, synchrotron beamlines, and VUV spectroscopy.
- Infrared Spectroscopy: Optical windows and components for FTIR and IR analytical instruments.
- X-Ray Diffraction (XRD): Optical elements and crystal blanks for X-ray diffraction and analytical devices.
- Cryogenic & Space Instrumentation: Sensors and optical windows operating in cryogenic or vacuum environments.
- Nuclear & Radiation Windows: Optical windows for high-radiation environments due to strong radiation resistance.
Shape Optics Supply Capability
- Optical elements for X-ray diffraction devices
- Optical components for IR, UV, and VUV applications
- High-quality LiF crystal blanks
- OEM and custom fabrication services
Manufacturing Capabilities
| Parameter | Specification |
|---|---|
| Maximum Diameter | Ø170 mm |
| Clear Aperture | ≥ 85% |
| Surface Flatness | λ/4 @ 633 nm |
| Surface Quality | 20/10 |
Optical Properties
| Property | Value |
|---|---|
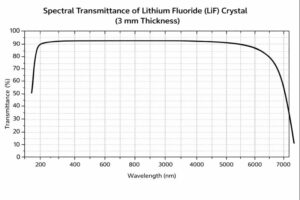
| Transmission Range | 0.105 – 6 µm |
| Transmittance | > 90% @ 0.3 – 4.5 µm |
| Refractive Index | 1.6240 @121 nm 1.3733 @2.5 µm |
| Reflection Loss | ~4.4% @4 µm (both surfaces) |
Physical Properties
| Property | Value |
|---|---|
| Density | 2.64 g/cm³ |
| Melting Point | 845 °C |
| Thermal Conductivity | 11.3 W·m⁻¹·K⁻¹ @314 K |
| Thermal Expansion | 37 × 10⁻⁶ K⁻¹ |
| Mohs Hardness | ~113 kg/mm² (600 g indenter) |
| Specific Heat Capacity | 1562 J·kg⁻¹·K⁻¹ |
| Dielectric Constant | 9.0 @100 Hz |
Mechanical & Elastic Properties
| Property | Value |
|---|---|
| Young’s Modulus (E) | 64.79 GPa |
| Shear Modulus (G) | 55.14 GPa |
| Bulk Modulus (K) | 62.03 GPa |
| Rupture Modulus | 10.8 MPa |
| Elastic Constants | C₁₁ = 112, C₁₂ = 45.6, C₄₄ = 63.2 |
Chemical & Crystal Properties
| Property | Value |
|---|---|
| Chemical Formula | LiF |
| Molecular Weight | 25.94 |
| Crystal Structure | Cubic |
| Cleavage Plane | (100) |
| Solubility | 0.27 g @18 °C |
Application Summary
Lithium Fluoride crystals are widely used as windows, lenses, and prisms for 105 nm to 6 µm optical systems and serve as critical components in X-ray diffraction and high-radiation optical devices. Proper handling and precision fabrication are essential due to LiF’s relative softness and cleavage characteristics.

